Abstract
Background: Patients with primary CNS Lymphoma (PCNSL) often present with neurologic complications such as focal defects, neuropsychiatric symptoms, increased intracranial pressure, aphasia, seizures, and ocular symptoms which require initiation of prophylactic or therapeutic anticonvulsants. Most anticonvulsants are notorious for their pharmacodynamic and pharmacokinetic drug interactions. The cornerstone of PCNSL therapy is the utilization of high-dose methotrexate (HD-MTX). The pharmacokinetics of HD-MTX are uniquely sensitive to both disease biology and drug interactions. Due to the importance of both HD-MTX and anticonvulsants in PCNSL patients, these medications are frequently given in concert with one another despite theoretical and proven drug interactions published in the literature. This is a retrospective analysis of patients treated with a modified R-MPV regimen (rituximab, methotrexate, procarbazine, vincristine alternating with intrathecal methotrexate) and the effects of various anticonvulsants on HD-MTX clearance.
Methods: Patients ≥18 years of age with a biopsy proven diagnosis of PCNSL at Roswell Park Comprehensive Cancer Center who received R-MPV between January 2002 and December 2019 were included in this retrospective analysis. The electronic health record of each patient was reviewed for specific patient demographics, laboratory results, confounding drug interactions, toxicity, and treatment outcomes. Descriptive statistics were computed for all categorical variables. The time to MTX clearance in hours and the potential impact of concomitant use of various anticonvulsants were assessed using the Wilcoxon Rank Sum test in the case of ordinal responses and the Pearson chi-square test or Fisher's Exact test for categorical variables where appropriate.
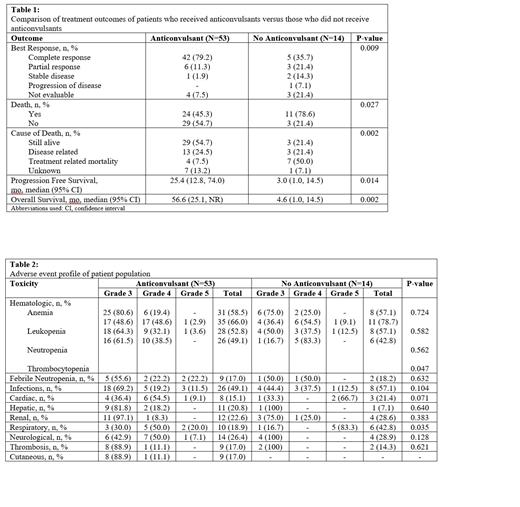
Results: A total of 67 patients met the inclusion criteria of which 53 patients (79.1%) received anticonvulsants (189 cycles of R-MPV) and 14 did not (69 cycles of R-MPV). Patients who had seizures or frontal brain PCNSL were more likely to receive anticonvulsants (p <0.001). When considering all possible medication interactions with HD-MTX, use of any interacting medication within 48 hours prior to HD-MTX was a predictor of delayed clearance (p = 0.0398). The most common confounding interaction seen in this population were with proton pump inhibitors. Levetiracetam was the most common anticonvulsant used at any point in time during treatment (83.1%). Compared to no anticonvulsant, levetiracetam did not significantly prolong HD-MTX clearance (52.6h v. 72.7h, p = 0.1586). Other anticonvulsants utilized included phenytoin (24.5%), gabapentin (3.8%), valproate (3.8%), carbamazepine (1.9%), lacosamide (1.9%), lamotrigine (1.9%), and phenobarbital (1.9%). Of the patients who received an anticonvulsant, 11 received more than one over the treatment period. Time to clearance of HD-MTX for patients not on an anticonvulsant was 53.8 hours compared to a clearance time of 68.2 hours for those who received an anticonvulsant (p = 0.0571). Patients who received anticonvulsants had a significantly higher complete response rate (79.2% vs 35.7%, p = 0.009), longer progression free survival (25.4 mo vs 3 mo, p = 0.014) and overall survival (56.6 mo vs 4.6 mo, p = 0.002) compared to those who did not (Table 1). There was no difference in grade 3/4 toxicities between anticonvulsants and no anticonvulsants except for thrombocytopenia (p = 0.047) and respiratory side effects (p = 0.035).
Conclusion: Anticonvulsants are an essential component to PCNSL therapy in patients on HD-MTX. They are well tolerated, do not alter HD-MTX clearance in a clinically significant manner and may help improve patient outcomes.
Torka: TG Therapeutics: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal